Recent Developments in the Diagnosis and Treatment of Pituitary Pars Intermedia Dysfunction (PPID)
Jo Ireland BVMS PhD Cert AVP(EM) MRCVS - 18/04/2014
Recent Developments in the Diagnosis and Treatment of Pituitary Pars Intermedia Dysfunction (PPID)
Jo Ireland BVMS PhD Cert AVP(EM) MRCVS
Pituitary pars intermedia dysfunction (PPID), also known as Equine Cushing’s Disease, is the most common endocrine disorder of older equids. Recent research studies have lead to improvements in the accuracy of diagnostic testing for PPID, and there is an increasing awareness, in both the equine veterinary profession and horse owners, of the welfare benefits of early diagnosis.
Clinical Signs of PPID
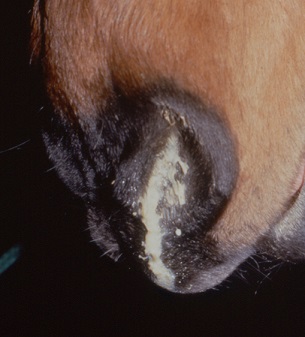
Clinical signs of PPID have been well documented and include hypertrichosis (commonly described as hirsutism), or more subtle hair coat abnormalities, laminitis, lethargy, weight loss and altered fat distribution, epaxial muscle wastage, polyuria and polydipsia, excessive or patchy sweating and increased susceptibility to infection [1,2]. While recurrent infections, such as solar abscesses or sinusitis, may be problematic, laminitis is the major health concern in cases of PPID and represents the most common reason for euthanasia.
Pasture Associated Endocrinopathic Laminitis

Pasture-associated laminitis is the most common form of laminitis reported by horse owners, and until recently, even within the veterinary profession, laminitis was frequently considered to be primarily a spring affliction associated with the ingestion of “lush” pasture. Traditional experimental models of laminitis, including starch and fructan overload models, induced disruption of the mucosal barrier in the colon and caecum resulting in systemic absorption of toxins that triggered laminitis. It was proposed that naturally-occurring cases of laminitis could be caused by consuming excessive amounts of fructan found in spring grass resulting in dysfermentation and acidosis in the hindgut.
To induce laminitis experimentally in a 300kg pony, the amount of fructan or starch required would be a bolus administration of 1.5 – 3kg [3-5]. During normal grazing, the fructan intake for a 300kg pony would range from 5 – 450g per hour, depending on appetite, grass type, climatic, temporal and environmental factors. Therefore the quantities of fructan, starch and simple sugars ingested by grazing animals are far lower than that required to damage the mucosal barrier and would not result in significant absorption of toxins from the hindgut. However, fructan or other non-structural carbohydrates ingested at a rate of 50 – 75g per hour cause marked hyperinsulinaemia [6,7], which is highly likely to further increase the risk of laminitis in animals with an underlying endocrinopathy, namely PPID or equine metabolic syndrome (EMS). These figures strengthen the hypothesis that, rather than gastrointestinal dysfunction, endocrinopathies are the underlying cause of “pasture-associated” laminitis.
 The other primary cause of endocrinopathic laminitis is Equine Metabolic Syndrome (EMS). EMS shares some attributes with Type II diabetes in humans, and is characterised by:
The other primary cause of endocrinopathic laminitis is Equine Metabolic Syndrome (EMS). EMS shares some attributes with Type II diabetes in humans, and is characterised by:
1. Being overweight and/or having abnormal fat distribution (this disorder can therefore occur in normal or even underweight animals).
2. Hyperinsulinaemia (although some EMS cases may have normal resting insulin concentrations)
3. Insulin resistance – a failure to respond normally to circulating insulin.
EMS is associated with laminitis, which can range from recurrent mild episodes in the spring and summer to severe laminitis that persists or recurs despite good management and veterinary treatment. Certain breeds are particularly susceptible to EMS, especially native British pony breeds, but the condition is also seen in many horse breeds.
Two studies have confirmed that approximately 90% of laminitis cases had one of these underlying endocrine disorders [8,9]. These research advances, together with the clinical observation that laminitis rarely occurs in young, fit, non-obese, non-native horses without signs indicative of an endocrinopathy, have resulted in a revolution regarding our understanding of laminitis in the UK equine population.
Hyperinsulinaemia, which is a characteristic feature of EMS and also found in a considerable proportion of PPID cases, has recently been shown to cause laminitis in horses and ponies [10,11]. Serum insulin concentration is an important prognostic indicator for both PPID and laminitis [12,13]. While the role of insulin in EMS-associated laminitis is now well recognised, historical beliefs surrounding PPID may have obscured the association between insulin and laminitis to some degree. The increased risk of laminitis with PPID was previously considered to result from high basal cortisol concentrations in affected animals, however there is little evidence to support a causative role of cortisol in laminitis and in fact cortisol has little diagnostic value in PPID cases as many have normal levels. Therefore, it is now considered that insulin is very relevant to the mechanisms underlying laminitis in PPID as well as in EMS cases, and may help to explain why some animals with PPID are more susceptible to laminitis than others.
Diagnosing PPID
In PPID, age-related dopaminergic neurodegeneration results in loss of dopaminergic inhibition leading to overproduction and elevated circulating levels a number of pro-opiomelanocortin (POMC)-derived peptides, including adrenocorticotrophic hormone (ACTH) [2]. Several endocrine tests have been employed in the diagnosis of PPID, of which the overnight dexamethasone suppression test (ODST) and basal plasma ACTH are the most frequently used.
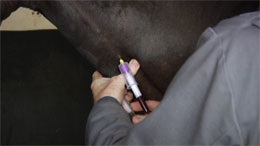
Reviewing previous studies showed that the ODST has an accuracy of approximately 88% [14-16], which compares favourably to the 90% accuracy reported for ACTH [16,17]. However, concern regarding the administration of exogenous glucocorticoids to laminitis-prone animals, from both veterinary surgeons and horse owners, together with the requirement for two blood samples obtained 18-24 hours apart and relative expense, mean that the ODST is now performed far less frequently than ACTH.
Although widely used, some potential issues regarding ACTH assays have been proposed. Many factors can elevate basal ACTH including exercise [18,19], stress [20], transport [21] and disease [22,23], although the effects of these factors have not been evaluated in PPID cases. In healthy horses, stress induced by 10 minutes of isolation did not significantly affect ACTH overall, however three of the five horses showed marked increases in ACTH concentration during isolation [20]. Cases of laminitis, acute abdominal disease and other acute or chronic diseases in a referral hospital study all showed modest increases in ACTH concentration compared to healthy controls [22]. Severe illness can result in prolonged elevation of ACTH, while mild to moderate disease may result in minimal elevations, which decline fairly rapidly to within reference range [23]. It is important to take these factors into consideration when using basal ACTH as diagnostic test for PPID, particularly in acute cases of laminitis.
Protocols for sample handling have previously been complicated by concerns regarding the labile nature of ACTH. Data from the Liphook Equine Hospital laboratory demonstrate that contrary to popular belief, ACTH degrades relatively slowly in EDTA plasma. In fact, even at room temperature, the average reduction in ACTH values after two days of storage was only 30% and the largest fall in any tested sample was 52%. Similarly, plasma ACTH levels were not significantly different between samples collected in glass or plastic tubes, or following chilled storage for two, four or eight hours prior centrifugation and separation of the plasma [24]. Additionally, no significant difference in ACTH level was measured from samples following up to three freeze-thaw cycles [24]. Based on these data, there appears to be no additional advantage of freezing plasma samples prior to laboratory submission, and ACTH is stable provided that the plasma sample is chilled within three hours and transported to the laboratory using a chill pack.
Seasonal changes in the equine hypothalamic-pituitary axis are now well documented, and earlier research suggested that there was a high risk of false positive PPID diagnostic test results during the autumn months. However, recently published findings show that while normal horses have increased ACTH levels in the autumn, the magnitude of this increase is far greater in PPID cases [25]. In fact, the diagnostic power of ACTH assays is greatest during the autumn months [26], as there is a more pronounced difference between diseased and healthy animals. As ACTH levels are significantly higher in the autumn compared to all other times of year, the development of seasonally adjusted reference ranges provided increased confidence in the accuracy of testing for PPID in the autumn months.
Treatment and Monitoring PPID

Prascend (pergolide) is the only licensed treatment available for PPID. A starting dose of approximately 2 μg/kg would be recommended regardless of ACTH levels prior to treatment, and using higher doses initially may increase the risk of potential adverse effects such as inappetence. Cases of PPID represent a wide spectrum of disease and pergolide dose should be adjusted for individuals if required based on clinical improvement and the results of further laboratory testing. Results of repeat samples submitted to Liphook’s laboratory showed that while ACTH may not return to normal levels, in most cases there is a significant reduction following treatment with pergolide. The reduction in ACTH appears to occur relatively rapidly after treatment commences, with approximately 50% of cases responding within the first week on pergolide and the vast majority demonstrating decreased ACTH after a month of treatment. Based on these data, ACTH assays represent a useful monitoring tool in the management of PPID cases. A practical approach would be obtaining a follow-up ACTH level one month after starting pergolide treatment, with subsequent dose alterations introduced slowly based on these results. Once ACTH levels appear controlled, regular monitoring at 3 – 12 month intervals is indicated. Pergolide dose should be titrated to the lowest effective dose in each case, but as PPID is a slowly progressive disease it is possible that some individuals may require increased medication over time.
Conclusion
Equine geriatric medicine continues to increase in importance. As the proportion of geriatric horses in our equine population increases, inevitably so will the number of PPID and endocrinopathic laminitis cases we treat. In all laminitis cases, the underlying cause needs to be identified and treated, and while the focus tends to be on EMS in younger animals, it is important to consider also testing for PPID, as the distinction between the two may not be clear and it is increasingly recognised that both conditions can occur concurrently. Recent data from Boehringer Ingelheim’s Talk About Laminitis disease awareness initiative which has facilitated the testing of over 20,000 horses for PPID in the past 3 years has revealed that over one third of laminitics aged 10-15 years have PPID, based on elevated basal ACTH. Improved understanding of diagnosis, treatment and monitoring for PPID in recent years will aid practitioners in successful case management, which in turn should improve the quality of service we offer to owners, who often have a very strong bond with their elderly horse and are keen to provide the best possible care.
This article was kindly sponsored by Boehringer Ingelheim, makers of Prascend ®:

References
1] McCue, P.M. (2002) Equine Cushing’s disease. Vet. Clin. N. Am.: Equine Pract. 18, 533-543.
2] McFarlane, D. (2011) Equine pituitary pars intermedia dysfunction. Vet. Clin. N. Am.: Equine Pract. 27, 93–113.
3] French, K.R. and Pollitt, C.C. (2004) Equine laminitis: loss of hemidesmosomes in hoof secondary epidermal lamellae correlates to dose in an oligofructose induction model: an ultrastructural study. Equine Vet. J. 36, 230-235.
4] Crawford, C., Sepulveda, M.F., Elliott,J., Harris, P.A. and Bailey, S.R. (2007) Dietary fructan carbohydrate increases amine production in the equine large intestine: implications for pasture-associated laminitis. J. Anim. Sci. 85, 2949- 2958.
5] Tóth, F., Frank, N., Chameroy, K.A. and Bostont, R.C. (2009) Effects of endotoxaemia and carbohydrate overload on glucose and insulin dynamics and the development of laminitis in horses. Equine Vet. J. 41, 852-858.
6] Bailey, S.R., Menzies-Gow, N.J., Harris, P.A., Habershon-Butcher, J.L., Crawford, C., Berhane, Y., Boston, R.C. and Elliott, J. (2007) Effect of dietary fructans and dexamethasone administration on the insulin response of ponies predisposed to laminitis. J. Am. Vet. Med. Assoc. 231, 365-373.
7] Frank, N., Elliott, S.B., Chameroy, K.A., Tóth, F., Chumbler, N.S., McClamroch, R. (2010) Association of season and pasture grazing with blood hormone and metabolite concentrations in horses with presumed pituitary pars intermedia dysfunction. J. Vet. Intern. Med. 24, 1167-1175.
8] Donaldson, M.T., Jorgensen, A.J. and Beech, J. (2004) Evaluation of suspected pituitary pars intermedia dysfunction in horses with laminitis. J. Am. Vet. Med. Assoc. 224, 1123-1127.
9] Karikoski, N.P., Horn. I., McGowan. T.W. and McGowan, C.M. (2011) The prevalence of endocrinopathic laminitis among horses presented for laminitis at a first-opinion/referral equine hospital. Domest. Anim. Endocrinol. 41, 111-117.
10] Asplin, K.E., Sillence, M.N., Pollitt, C.C. and McGowan, C.M. (2007) Induction of laminitis by prolonged hyperinsulinaemia in clinically normal ponies. Vet. J. 174, 530-535.
11] de Laat, M.A., McGowan, C.M., Sillence, M.N. and Pollitt, C.C. (2010) Equine laminitis: Induced by 48 h hyperinsulinaemia in Standardbred horses. Equine Vet. J. 42,129-135.
12] Walsh, D.M., McGowan, C.M., McGowan, T.W., Lamb, S.V. Schanbacher, B.J. and Place, N.J. (2009) Correlation of plasma insulin concentration with laminitis score in a field study of Equine Cushing’s Disease and Equine Metabolic Syndrome. J. Equine Vet. Sci. 29, 87-93.
13] McGowan, C.M., Frost, R., Pfeiffer, D.U. and Neiger, R. (2004) Serum insulin concentrations in horses with equine Cushing’s syndrome: response to a cortisol inhibitor and prognostic value. Equine Vet. J. 36, 295–298.
14] Dybdal, N.O., Hargreaves, K.M., Madigan, J.E., Gribble, D.H., Kennedy, P.C. and Stabenfeldt, G.H. (1994) Diagnostic testing for pituitary pars intermedia dysfunction in horses. J. Am. Vet. Med. Assoc. 204, 627-632.
15] Frank, N., Andrews, F.M., Sommardahl, C.S., Eiler, H., Rohrbach, B.W., Donnell, R.L. (2006) Evaluation of the combined dexamethasone suppression/thyrotropin-releasing hormone stimulation test for detection of pars intermedia pituitary adenomas in horses. Vet. Intern. Med. 20, 987-993.
16] Beech, J., Boston, R., Lindborg, S. and Russell, G.E. (2007) Adrenocorticotropin concentration following administration of thyrotropin-releasing hormone in healthy horses and those with pituitary pars intermedia dysfunction and pituitary gland hyperplasia. J. Am. Vet. Med. Assoc. 231, 417-426.
17] van der Kolk, J.H., Wensing, T., Kalsbeek, H.C. and Breukink, H.J. (1995) Laboratory diagnosis of equine pituitary pars intermedia adenoma. Domest. Anim. Endocrinol. 12, 35-39.
18] Alexander, S.L.; Irvine, C.H.G.; Ellis, M.J. and Donald, R.A. (1991) The effect of acute exercise on the secretion of corticotropin-releasing factor, arginine vasopressin, and adrenocorticotropin as measured in pituitary venous blood from the horse. Endocrinology 128, 65-72.
19] Marc, M., Parvizi, N., Ellendorff, F., Kallweit, E. and Elsaesser, F. (2000) Plasma cortisol and ACTH concentrations in the warmblood horse in response to a standardized treadmill exercise test as physiological markers for evaluation of training status. J. Anim. Sci. 78, 1936-1946.
20] Alexander, S.L., Irvine, C.H.G., Livesey, J.H. and Donald, R.A. (1988) Effect of isolation stress on concentrations of arginine vasopressin, alpha-melanocyte-stimulating hormone and ACTH in the pituitary venous effluent of the normal horse. J. Endocrinol. 116, 325-334.
21] Fazio, E., Medica, P., Aronica, V., Grasso, L. and Ferlazzo, A. (2008) Circulating β-endorphin, adrenocorticotrophic hormone and cortisol levels of stallions before and after short road transport: Stress effect of different distances. Acta Vet. Scand.50: 6.
22] Ayala, I., Martos, N.F., Silvan, G., Gutierrez-Panizo, C., Clavel, J.G., and Illera, J.C. (2012) Cortisol, adrenocorticotropic hormone, serotonin, adrenaline and noradrenaline serum concentrations in relation to disease and stress in the horse. Res. Vet. Sci. 93, 103-107.
23] Towns, T.J., Stewart, A.J., Hackett, E., Zhong, Q., Munsterman, A., Wooldridge, A.A., Funk, R.A. and Hewes, C.A. (2010) Cortisol and ACTH concentrations in ill horses throughout 6 days of hospitalization. J. Vet. Emerg. Crit. Car. 20, A16.
24] Perkins, G.A., Lamb, S., Erb, H.N., Schanbacher, B., Nydam, D.V. and Divers, T.J. (2002) Plasma adrenocorticotropin (ACTH) concentrations and clinical response in horses treated for equine Cushing's disease with cyproheptadine or pergolide. Equine vet. J. 34, 679-685.
25] Copas, V.E.N. and Durham, A.E. (2011) Circannual variation in plasma adrenocorticotropic hormone concentrations in the UK in normal horses and ponies, and those with pituitary pars intermedia dysfunction. Equine Vet. J. 44, 440-443.
26] McGowan, T.W., Pinchbeck, G.P. and McGowan, C.M. (2013) Evaluation of basal plasma α-melanocyte-stimulating hormone and adrenocorticotrophic hormone concentrations for the diagnosis of pituitary pars intermedia dysfunction from a population of aged horses. Equine vet. J. 45, 66-73.