Equine tendon injury in the 21st century: Old treatments fired and stem cells promoted?
Dr Charlotte Avella and Professor Roger Smith - 08/01/2011
Equine tendon injury in the 21st century:
Old treatments fired and stem cells promoted?
Introduction
Tendon injury is significant in horses due to its common occurrence, the lack of a highly effective treatment, the requirement for lengthy rehabilitation, and the risk of reinjury. In horses the superficial digital flexor tendon (SDFT) of the forelimb is the most commonly injured tendon. Tendinopathy is most common in racehorses and event horses, but can occur in horses used for any discipline. A recent study of 148 National Hunt racehorses in training, at ten training yards in the UK, revealed a 24% prevalence of SDFT tendinopathy detected using ultrasonography (Avella et al., 2009).
Figure 1: Tendon injuries are most common in racehorses and eventers.
Diagnosis of tendon injury
Pathophysiology of tendon injury and repair
Once the peak load on the tendon overcomes its structural strength, there is physical disruption to the tendon matrix. The clinical injury varies in degree from fibrillar slippage, with breakage of crosslinking elements, to fibrillar rupture and, in some cases, complete separation of tendon tissue. Once this occurs, the damage created induces a repair process that results in inflammation followed by fibroplasia. Thus synthesis of scar tissue occurs that has a composition which is different from that of tendon, with a higher ratio of type III:I collagen (about 50% compared with 10% for normal tendon), a higher hydration, and higher levels of glycosaminoglycans (GAGs) (Birch et al., 1998). Although mature scar tissue tends to be less stiff as a material than tendon, because large amounts are formed, the scarred tendon often results in a stiffer tendon than the original. As a result, the healed tendon becomes strong but it is functionally inferior to normal tendon predisposing it to re-injury, often at sites adjacent to the original injury.
Treatment of tendon injury
In 1964, Asheim described the common treatment of tendon and ligament injuries as “phlebotomy, local cooling, plaster bandaging and rest (Asheim 1964). Over the last 4 decades, many treatments have been proposed for the management of tendon injury, although few, if any, have convincing supporting evidence of efficacy, and some can even be considered deleterious or detrimental to healing. However, the basic principles described by Asheim of cooling, support, and rest remain integral parts of the management protocol.
New advances: Stem cell therapy
The hypothesis for stem cell therapy for the treatment of tendon injuries is that implanted bone marrow-derived mesenchymal stromal cells (MSCs) will be capable of synthesising matrix more similar to tendon and less like scar tissue, to provide a superior functional outcome. An initial safety trial was executed to provide proof of concept. Six horses that had moderate to severe SDFT injuries were recruited onto the trial which demonstrated that the MSCs therapy did not have adverse effects such as formation of inappropriate tissue.
The use of MSCs for intralesional treatment of tendinopathy offers the prospect of tissue regeneration rather than repair. MSCs have the potential to differentiate into tenocytes and to regenerate tendon matrix, thereby creating a repair that is functionally superior to fibrous scar tissue. Initial studies have included the isolation of MSCs from either bone marrow or fat, followed by either direct implantation or expansion in vitro before implantation (Dahlgren et al., 2002).
Technique
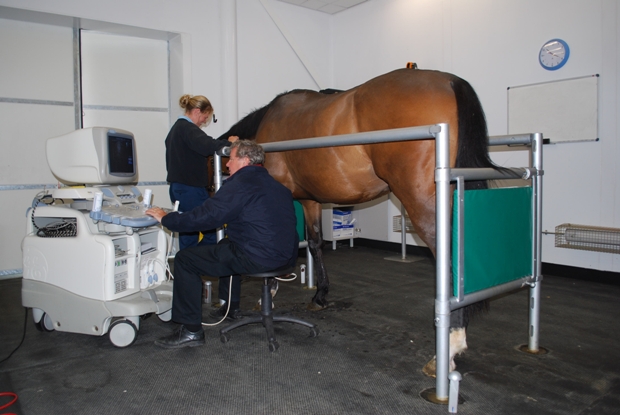
Bone marrow (BM) is recovered from the sternum following the diagnosis and assessment of tendon injury. Using standing sedation and local anaesthesia, the inter-sternal space is identified using ultrasonography, and the BM from the sternebrae either side is aspirated using a Jamshidi needle. The BM derived cells are cultured in vitro and the nucleated adherent cell population are recovered and resuspended in citrated BM supernatant. Implantation is performed at approximately 2-3 weeks after BM aspiration. The horse is sedated and local regional analgesia of the palmar metacarpal soft tissues is performed. The MSCs are injected at specific sites along the tendon depending on the extent of the injury. The implantation of the MSCs is performed using ultrasound guidance. Figure 3 shows Professor Roger Smith performing an ultrasound examination at the Royal Veterinary College Equine Referral Hospital.
Ultrasonographic assessment of outcome
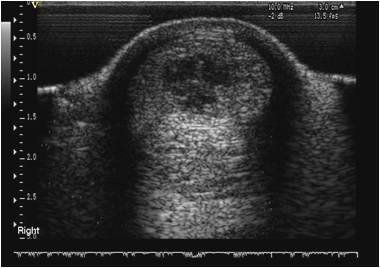
Horses with tendon injuries treated with stem-cell therapy have demonstrated rapid ultrasonographic improvement of lesions. This includes increased echogenicity in the initially hypoechoic lesion reaching similar echogenicity to normal tendon over several months. In addition to improved density of the fibre pattern, evidence of lengthening of the linearly arranged fibres was apparent. The sequential ultrasound examinations have provided evidence of good to satisfactory healing following stem cell therapy.
Functional outcome
The long term outcome of stem cell therapy is assessed by clinical examination, the presence of ultrasonographic evidence of tendon repair and ultimately by whether the horse re-injures. Prevention of re-injury is the primary aim of tendon therapy. To date over 1500 horses with SDFT tendinopathy have been treated with intralesional autologus MSCs. Analysis of the clinical outcome in 113 national hunt racehorses with 2 years of follow up post-treatment gave a reinjury rate of 28.9% (Smith 2010). This reinjury rate is significantly better than data previously reported for a similar population of NH horses treated conventionally and analysed in the same way (56%) (Dyson et al., 2004). Conventional therapies with which stem cell therapy was compared included controlled exercise alone, or controlled exercise combined with intra-lesional tendon treatments of hyaluronic acid or polysulphated glycosaminoglycans (PSGAGs).
Conclusion
The use of bone marrow derived autologus mesenchymal stromal cells intralesionally to treat tendon injury appears to significantly decrease the risk of reinjury, thus potentially extending the athletic career of the horse and reducing morbidity.
This article was provided by VetCell, for more information please contact VetCell on 01865 922227, email: info@vetcell.com or visit www.VetCell.com:

References
Asheim A: Surgical treatment of tendon injuries in the horse, J Am Vet Med Assoc, 145: 447, 1964.
Avella, C. S., Ely, E. R., Verheyen, K. L. P., Price, J. S., Wood, J. L. N. & Smith, R. K. W. 2009. Ultrasonographic assessment of the superficial digital flexor tendons of National Hunt racehorses in training over two racing seasons Equine Vet J. 2009 May;41(5):449-54.
Birch, H. L., Bailey, A. J. & Goodship, A. E. 1998. Macroscopic 'degeneration' of equine superficial digital flexor tendon is accompanied by a change in extracellular matrix composition. Equine Vet J, 30, 534-9.
Dahlgren LA, van der Meulen MCH and Bertram JEA et al: Insulin-like growth factor-I improves cellular and molecular aspects of healing in a collagenase-induced model of flexor tendinitis. Journal of Orthopaedic Research, 20:910, 2002.
Smith 2010 ‘Bone marrow-derived stem cells for tendon and ligament repair. P119-120 proceedings of the British Equine Veterinary Association Congress September 2010.