Show all clinical related articles
Tips for Safer & Less Stressful Small Animal Anaesthesia
Matthew McMillan BVM&S MRCVS Senior Clinical Training Scholar in Anaesthesia and Analgesia - 10/08/2021
Tips for safer and less stressful small animal anaesthesia;
Proper preparation prevents poor performance
There are two underlying fundamentals which should be paramount in your mind whilst administering any anaesthetic:
- Ensuring the patient recovers fully and rapidly with minimal side effects.
- Ensuring the patient receives appropriate analgesia and is as pain free as possible.
Remember, every anaesthetic has the potential to cause harm if not adequately prepared for and then carefully monitored. It is the proper preparation of anaesthetic equipment, the patient and the anaesthetist that reduces the chances of problems occurring and makes them easier to deal with when they do!
Equipment Checks
In the same way that a pilot needs to check a plane before takeoff, an anaesthetist needs to check the equipment they plan to use before using it. Although true equipment failure in anaesthesia is rare, an unnervingly high proportion of critical incidents (occurrences that have the potential to or actually do cause injury to a patient) during anaesthesia occur due to the anaesthetic machine and/or breathing system not being adequately checked. Common problems include inadequate oxygen levels in oxygen cylinders, lack of a back up oxygen source, leaving the breathing system APL valve closed, semi obstructed endotracheal tubes, and endotracheal tube cuffs that deflate. The anaesthetic machine, breathing system and monitoring equipment should all be included in this “pre-flight” check.
Checking anaesthetic equipment should be performed in an organised and consistent manner. The process can be streamlined and aided by the use of a checklist. The role of a checklist is to outline the expected steps that need to be carried out in order to perform a procedure- in this case the checking of the anaesthetic equipment. After many anaesthetics this procedure may become second nature but forgetting a step is relatively easy (even for the most seasoned of anaesthetists!). The use of checklists has, unsurprisingly, been found to improve memory recall especially in mundane matters that may become easily overlooked in stressful situations. It also reduces the urge to cut corners!
Below is an example of an anaesthetic machine checklist. In order to perform a check you will have to get to know the anaesthetic machines and monitoring equipment you have available. Also it is important to know where the spare oxygen cylinders are kept!
ANAESTHETIC MACHINE CHECKLIST
- Connect any electrical equipment to the mains
- Check oxygen supplies, make sure adequate back up supplies are readily available, ensure cylinders are mounted properly and are not leaking
- Check oxygen alarm
- Check flow meters
- Check vaporiser is mounted properly on back bar
- Check the vaporiser dial turns smoothly and doesn’t stick
- Check level of volatile anaesthetic agent in vaporiser
- Attach breathing system to common gas outlet and to scavenging system
- Check breathing system, anaesthetic machine and vaporiser for leaks
- Check APL valve (ensure that the valve is left open)
- Check scavenging system (weigh Cardiff aldosorbers, check airbrake working etc)
- Check monitoring equipment turns on and is working and is set up suitable to use on the next patient
Anaesthetic planning
The objective of your anaesthetic plan is to set out beforehand what drugs and equipment you will need and to outline the most likely potential complications and have a plan ready for treating them. You should plan to provide a balanced anaesthetic. The objectives of balanced anaesthesia being; patient stress reduction, provision of appropriate analgesia, and reduction of adverse effects associated with anaesthetic drugs.
Assess your patient
The first step in designing an anaesthetic plan is to assess the patient; this includes assessment of the patient’s history and clinical examination. The following assessments and questions should be forefront in your mind;
Demeanour and behaviour - Is the patient stressed? Aggressive? Depressed? Excitable? How easy will it be to restrain? This will help establish what level of sedation is required in the premed.
Cardiovascular status - Does the patient have a normal volume status? Are the heart rate, capillary refill time, mucous membrane colour and pulse quality within normal limits? Does the patient have an arrhythmia? A heart murmur? History of exercise intolerance? This may affect your choice of anaesthetic drugs. You may want to have your patient attached to an ECG during the induction and anaesthesia and check the patient’s blood pressure before you start. You should also consider pre-oxygenating your patient.
Respiratory system - Does the patient have a normal/appropriate breathing pattern and respiratory rate? Does the patient have appropriate lung sounds throughout all areas of the chest? Is an upper airway problem likely? Is endotracheal intubation likely to be routine or problematic? In cases where upper airway problems are possible intubation aids might be needed (described later). Patients with pulmonary disease may need ventilating either with a mechanical ventilator or by hand in order to maintain oxygenation. Is a ventilator available and set up? Is the nurse appropriately trained in IPPV? Do I have capnography available? Most mechanical ventilation without capnography leads to hyperventilation. Again pre-oxygentaion is warranted for animals with history or clinical signs of respiratory disease.
Neurological - Are there any brain, neuromuscular or spinal problems? Is there a risk of hypoventilation or raised intracranial pressure? Patients with brain disease often require lower doses of drugs. You should also consider ventilating these patients as reducing CO2 levels helps reduce intracranial pressure (try maintaining patients at an ETCO2 of 35mmHg).
Abdominal - Does the animal have abdominal enlargement, a fluid thrill, abdominal pain? Abdominal enlargement can have cardiovascular and respiratory effects leading to hypotension and hypoventilation. Again mechanical ventilation may be required and blood pressure should be monitored.
Metabolic - Is there any evidence of electrolyte abnormalities, renal problems, hepatic problems? Is the patient likely to metabolise drugs normally? If not then use short acting and/or reversible drugs. Does the patient have low albumin? Will drug binding be an issue? If so you may need lower doses of drugs so use drugs that can be easily titrated to effect.
Body condition - Is the patient fat or thin or normal? Extremes of body condition can affect the distribution of drugs. Obesity can cause cardiovascular and respiratory problems that can be exacerbated under anaesthesia.
Global assessment - Do any of the findings highlight any specific risk factors? Do any of the problems found require stabilisation prior to anaesthesia (i.e. hypovolaemia or dehydration requiring fluids, electrolyte abnormalities such as hyperkalaemia requiring normalising)? Are any drugs contra-indicated? For example acepromazine is contra-indicated in patients at high risk of hypotension i.e. a patient with a GDV or ruptured spleen.
Once you have done this you should have a pretty good idea of the patient’s health status and any specific risk factors your patient has regarding anaesthesia. Anything you can do in order to sort out or improve problems before anaesthesia should be done.
Assess possible/probable complications of the anaesthesia and surgery
Once you have assessed the patient you need to assess the potential complications of the procedure and the anaesthesia. Alongside procedure specific problems there are a number of complications common to all anaesthesia and surgery. Below the causes and potential treatments are briefly outlined.
Complications of anaesthesia:-
Hypotension- Hypotension is extremely common under anaesthesia. In order to treat hypotension you first have to diagnose it. In an ideal world all anaesthetised patients would have blood pressure monitoring but in reality this may not be possible however blood pressure monitors are becoming more and more affordable and available so their use is on the rise (in fact many practices have a Doppler blood pressure unit which is often not used for anaesthesia)! There are two main mechanisms that lead to hypotension in anaesthetised patients; hypovolaemia and the cardiovascular effects of the anaesthetic drugs administered- most commonly vasodilation. Hypovolaemia is often secondary to blood loss during surgery, but underlying pre-operative volume deficits may be exacerbated by anaesthesia. Fluid therapy is the treatment of choice. Pre-operative fluid deficits should be corrected prior to induction. Which fluid and how much fluid to give remains controversial and much debated but basically you are looking at providing enough fluid to return your patient’s cardiovascular status to normal. In the initial stages of hypovolaemia most patients will compensate for a decrease in circulating volume by increasing their heart rate; blood pressure changes will occur at later stages. Therefore don’t rely solely on blood pressure but try to assess heart rate, capillary refill time, mucus membrane colour and pulse quality as well. A 10ml/kg bolus of crystalloid (i.e. Hartmann’s) or 2ml/kg of a colloid can be given over 5-10minutes and then the patient can be reassessed, multiple boluses maybe required. Vasodilation can be minimised by balanced anaesthesia which helps reduce the amount of anaesthetic drugs, specifically induction and inhalant anaesthetics, required. With any patient that is found to be hypotensive is worthwhile trying to turn down the vaporiser setting as small decreases in anaesthetic concentration can make drastic improvements this can be aided by improving the patient’s analgesia.
Hypothermia- Anaesthesia invariably effects temperature regulation. In a majority of cases this will manifest itself as hypothermia but during the summer large hairy dogs may well overheat. Small animals with large surface area to volume ratios lose heat extremely rapidly under anaesthesia and sedation but all animals may develop clinically relevant hypothermia. Hypothermia has many physiological effects and will delay greatly a patient’s recovery from anaesthesia. Care should be taken to maintain a patient’s temperature to as close to normal under all anaesthetics. Heat blankets (such as the Hot DogTM) or hot air blowers (such as the BairhuggerTM or CocoonTM) are very useful but not available in many practices. Techniques such as using heat pads, wrapping extremities with bubble wrap or silver foil, using hot hands (gloves filled with warm water), heated “wheatie” bags or gel packs, warming IV fluids etc can all be employed. If anything warm is to be placed next to an unconscious animal care should be taken as thermal burns are possible. Try to get into the habit of regularly checking an animal’s temperature under anaesthesia.
Hypoventilation- Respiratory depression is an adverse effect of most general anaesthetic drugs, including inhaled anaesthetics. This depression is caused by a decreased sensitivity to arterial CO2 levels and causes a reduction in minute volume by reducing tidal volume and/or respiratory rate. Capnography can be used to monitor ventilation in anaesthetised patients by measuring expired CO2. Normal end tidal CO2 levels are between 35-45mmHg but hypoventilation under anaesthesia will increase this. Where a capnograph is not available respiratory rate and an assessment of the movement of the patient’s rebreathing bag can be used to assess ventilation. Intermittent positive pressure ventilation is the treatment of choice for hypoventilation but reducing the doses of drugs (i.e. turning down the vaporiser setting) that cause respiratory depression should also improve ventilation.
Hypoxaemia- Hypoxaemia can occur in healthy animals under anaesthesia if oxygen supplementation is not administered. This is due to the effect of hypoventilation which leads to the build up of CO2 within the alveoli reducing the available space for oxygen. Because of this provision of 30% oxygen or more is recommended for anaesthesia. Atelectasis, collapse of alveoli in the periphery of dependent lung lobes, will affect pulmonary function under anaesthesia although we do not yet know the best way to manage this problem in anaesthetised patients, performing IPPV and administering a sigh (a large positive pressure breath held for 10-15 seconds) may be useful to recruit collapsed alveoli and might improve oxygenation.
Complications of surgery:-
Haemorrhage- Blood loss quickly builds up especially in smaller patients. Try to estimate blood loss where you can. Techniques include weighing and count swabs and checking the volumes in any suction bottles (remember to minus any flush solution used). Treatment requires replacement of the lost blood with fluid therapy. Crystalloids such as Hartmann’s (lactated Ringers solution or compound sodium lactate) or 0.9% NaCl (normal saline) don’t stay in the circulation for long tending to redistribute from the intravascular space into the interstitial space. Books tend to describe giving 2-3 times the volume of crystalloid as compared to blood lost whereas an equal volume of colloid is generally required. If estimation of blood loss is not possible then administration of 10ml/kg boluses of crystalloid or 2ml/kg of colloid can be administered until the patients cardiovascular parameters are returned to normal.
0-10% blood volume minimal blood loss
10-20% blood volume moderate blood loss
20-30% blood volume severe blood loss
>30% blood volume extreme blood loss
Remember blood volume is 90ml/kg in a dog and 60-70ml/kg in a cat
Colloids and blood transfusions should be considered as blood loss becomes more severe but up to approximately 30% blood loss can probably be treated with isotonic crystalloids in a previously healthy patient where other products are not available or affordable.
Pain- all surgical procedures involve some degree of pain, and each individual will respond differently to this pain. Therefore an analgesic plan needs to be individualised to each patient and each surgery. Saying that a number of questions can be asked about every patient, these include:
What drugs do I have available? Opiates, NSAIDs, local anaesthetics, ketamine......
Can I give an opiate? If so which opiate is most appropriate? Buprenorphine (0.02-0.04mg/kg) can be an adequate analgesic for most general practice procedures if administered incorporated into a multimodal analgesic plan. Methadone and morphine are probably superior analgesics but are not licensed and are less commonly available in general practice*. Make the most of them if you have access to them but remember they may not be suitable in all cases.
Can I give a non-steroidal anti-inflammatory drug (NSAID)? Does my animal have any history of receiving an NSAID? I like to stay with a previously administered drug if no side effects were seen. Does the animal have any reason to avoid giving NSAIDs? Has the patient got clinical signs or history of gastrointestinal disease, concurrent steroid administration, renal compromise, hypovolaemia or hypotension? If it does you probably shouldn’t administer an NSAID.
Can I use a local anaesthetic technique? Learn local anaesthetic techniques such as epidurals and nerve blocks as they can be very effective, requiring little equipment and are generally extremely cheap. Even splash, line and ring blocks can make a considerable contribution to a patient’s analgesia. Dental blocks are simple and can make a real impact when performing tooth extractions.
What can we do if the patient breaks through our initial analgesia? Ketamine and lidocaine (without adrenaline) constant rate infusions can be considered if equipment such as syringe driver or fluid pump is available but single dose techniques are described. Alpha-2 agonists such as medetomidine and dexmedetomidine can augment opiate analgesia.
What analgesia will the patient need post operative and how will I assess the patient? How will this analgesia be administered? Do I need to keep a catheter in the patient postoperatively? Is the patient likely to eat? So are oral drugs (NSAIDs, tramadol, gabapentin) an option? Do I have fentanyl patches available?
Preparation
Now you have an anaesthetic plan formed in your mind (or on a sheet of paper) organise all the equipment needed prior to anaesthesia, it’ll help streamline the process and means you instinctively know where things are. Have everything set out neatly on a surface or even on the anaesthetic machine, basically anywhere within easy reach of where you intend to induce the patient, left to right in the order you plan to use it. Calculate drug doses, flow rates etc and write them down on an anaesthetic record before you anaesthetise the patient. A checklist can be useful here too, laminate one that can be filled in and then wiped clean for each patient. Always label drugs, administering a transparent induction agent after confusing it with a saline flush solution could be disastrous.
Communication
You probably will not be monitoring the majority of the patients you anaesthetise personally, that role being taken by a veterinary nurse, therefore the anaesthetic plan should be discussed with the person or team of people that is going to be involved in the procedure.
- Discuss your anaesthetic plan and how you hope the procedure will proceed
- Discuss the potential problems and how you plan to treat them if they occur
- Set limits for parameters (for example say you want to be informed if you Doppler systolic blood pressure drops to below 80mmHg or rises above 140mmHg)
- Make sure the rest of the team are happy with the plan and don’t have any concerns or questions
Forming a good working relationship with your team by giving veterinary nurses responsibly and including them in the case management is vital. Clearly, for things you do every day, you will develop a routine but for new procedures or sick patients this process, although it may seem a little long winded, will help the anaesthetic proceed in a smooth manner.
Record keeping
It is a legal requirement to keep a record of all anaesthetics unless they are emergencies or extremely short (the RCVS do not give a definition of short). 5-10min assessment of at least depth, pulse rate/heart rate and RR would be considered the minimal acceptable monitoring. Preferably BP and ETCO2, SpO2 should also be monitored. Fill in the details of your anaesthetic record prior to induction of anaesthesia so the record can begin immediately post induction. All drugs given during the perioperative period, their doses and time of administration should be included on this record.
Only once the anaesthetic plan has been established, the anaesthetic machine and monitoring equipment checked and all the necessary equipment has been set out are you are ready to anaesthetise the patient. Getting used to performing this procedure on routine patients will make it second nature and will therefore improve the way you deal with an emergency situation.
Premedication
Appropriate premedication is a vital part of balanced anaesthesia. Premedication......
- Reduces stress - reduces stress related catecholamine effects such as hyper-excitation, fear aggression, tachycardia, hypertension
- Eases restraint and patient handling
- Reduces the dose of induction agent required - which reduces side effects of the induction agents and reduces the cost of induction
- Reduces the amount of maintenance agent required for a set level of anaesthesia
- Smoothes out recovery
Almost all animals benefit from some premedication prior to anaesthesia. Sick animals may just require an opiate (+/- a benzodiazepine) but healthy animals, especially when lively, stressed or aggressive will benefit from an opiate plus acepromazine or medetomidine.
Including an opiate alongside a sedative
- Reduces the dose of other drugs required to get a certain level of sedation so therefore reduces the side effects of that drug
- Gives a background level of analgesia
In patients that have a catheter preplaced premedication can be given intravenously (IV) however in animals with no IV access the intramuscular (IM) route is more convenient, therefore learning to give true intramuscular injections is extremely useful. There is a tendency to use short 5/8ths, small gauge (24g orange) needles to give IM injections because animals are seen to react less. This is probably fallacy as in many animals a 5/8ths needle will not make it into muscle but will deposit your injection into SC fat! Although this is non-painful it is also useless as drug distribution from SC fat is poor so your patient may not get any benefit out of the premed (and remember the premed may contain most of the patient’s analgesia as well as the sedative). A 1” 22g (blue) needle is likely to be adequate in most patients if using the muscles of the neck or the quadriceps, but even this may not be long enough for an overweight patient if using the muscles of the back! Quadriceps injection has been found to be more painful than other IM injection sites so should be used with care. Time and effort spent restraining an animal appropriately and giving a true IM injection will save time and stress when it comes to induction.
Induction
The old adage that the safest drugs are the ones you are used to is true. Ask yourself - Is there any reason not to use my normal induction agent? Is there any way of reducing the amount of induction agent I might need in higher risk patients? I.e. co-induction with diazepam or midazolam.
Another thing that greatly helps during the induction of anaesthesia is having good IV access. IV catheterisation eases induction by allowing drugs to be given at an appropriate rate. There is a tendency to rush the administration of off-the-needle drugs which may cause inadvertent overdose and leads to a higher incidence of side effects such as apnoea and hypotension. Also it makes giving top-ups of induction agent easier. In addition to this maintaining IV access during anaesthesia allows:
- rapid and easy administration of anaesthetic and emergency drugs
- administration of IV fluids
Both should improve patient safety and anaesthetist peace of mind!
Cost is normally the argument against placing IV catheters in practice, however lower doses of propofol and alfaxalone can be used when they are administered over 45-60 seconds so the cost can be partially, if not completely, recuperated. Using an appropriately sized syringe will also help. For example a 4mg/kg dose of propofol for a 30kg dog is 120mg or 12ml, this could all be drawn up in a 20ml syringe but a 20ml syringe is unwieldy and it is much harder to depress the plunger in a regular manner than a 10ml syringe.
If your patient gets stressed during IV catheterisation wait a few minutes before inducing anaesthesia and potentially consider giving additional premedication IV.
After induction apnoea may occur potentially leading to desaturation and hypoxaemia. Pre-oxygenation, the provision of supplemental oxygen prior to induction, prolongs the time it takes for haemoglobin to desaturate by about 2-3 minutes. Any sick or at risk animal (i.e. C-section or brachycephalic dog) should be preoxygenated for 5-10 minutes before induction, ideally after the IV catheter has been placed.
Intubation
Cats are much harder to intubate than dogs so I have concentrated on an explanation of intubating cats. Cats’ larynxes are very delicate and easily damaged so direct observation and proper technique should be employed during orotracheal intubation in cats in an attempt to avoid laryngeal trauma. A laryngoscope is invaluable here, you can intubate a cat without laryngoscopy but the visualization is poor and therefore the risk for trauma is much greater. If your practice doesn’t have one, consider investing in one yourself as the time and stress it will save is invaluable. There are various models available from most veterinary equipment suppliers. Alternatively a good light source and a tongue depressor could be used (head torches can be useful).
- Administer induction agent until a suitable plane of anaesthesia is reached - loss of jaw tone and minimal gag reflex - this is much easier if an IV catheter has been placed as drugs can be given more slowly and top-ups are easier to administer.
- The cat should be held with its head and neck extended, initially as close to horizontal as possible.
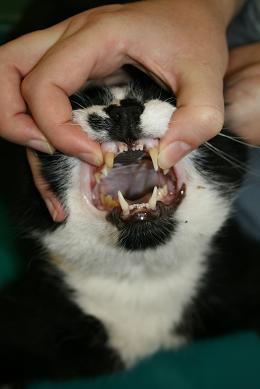
- Open the cat’s mouth and gently pull out the tongue between the lower canines - Do this gently as the hypoglossal/glossopharyngeal nerves can be stretched leading to neuropraxia and problems with the tongue post-anaesthesia.
- Depress the base of the tongue with a laryngoscope blade, this will disengage the soft plate and allow visualisation of the larynx. Do not depress the epiglottis as although this will allow good visualisation this can cause damage leading to swelling and laryngeal spasm.
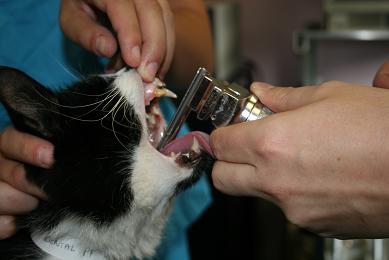
- Spray the larynx with a single spray of lidocaine. IntubezeTM should be held upright (with the spray nozzle parallel to the floor), test the spray before use to ensure the pump mechanism is loaded.
- Give at least 60-90 seconds for the local anaesthetic to have effect, it does not work immediately - initially the larynx may go into spasm due to irritation from the spray but once the local has worked the larynx should relax.
- During the waiting period the tongue should be relaxed and I generally remove the laryngoscope. Flow-by oxygen can be administered, and additional induction agent can be administered if required.
- Reposition the cat, with its head and neck extended, (to allow better visualisation and personal comfort this can be at more of an angle to the table than before). Use the laryngoscope as before to depress the tongue.
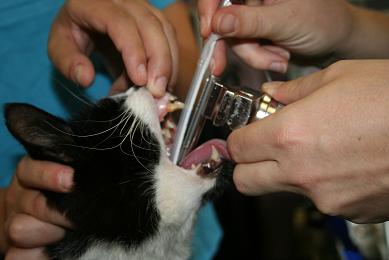

- Guide the ET tube over the epiglottis, aiming the bevel at the most ventral point of the laryngeal opening.
- Gently advance the tip of the ET tube through the laryngeal opening during inspiration (the laryngeal folds should abduct), a gentle twisting motion can be employed when using silicon tubes to aid passage.
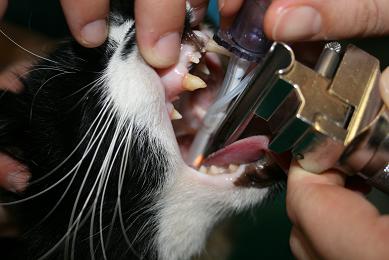
Tie the tube in place around the back of the head.
If using a stylet ensure that it does not protrude from the end of the ET tube as they can cause nasty damage to the larynx and trachea.
A fine relatively flexible urinary catheter slid through the lumen of an ET tube can be used as a guidewire to pass the ET tube over if necessary.
If using cuffed tubes be extremely careful when inflating the cuff. Never overinflate the cuff and be especially careful with red rubber tubes. One technique would be to listen for a leak at the cat’s mouth during a positive pressure breath and inflate until the leak can no longer be heard.
Remember that the cuff can cause shear damage to the wall of the trachea if twisted so during repositioning of the cat the ET tube should be disconnected from the breathing system when the cat is moved.
It may not be appropriate to intubate cats undergoing anaesthesia for very short procedures as intubation in these cases has been associated with higher mortality rates (CEPSAF, 2008) probably secondary to laryngeal and upper airway trauma. Supplemental oxygen can be administered via facemask; in fact a good facemask for a cat will also allow relatively effective short term IPPV if required.
In general dogs are far simpler to intubate and do not normally require a local anaesthetic spray. Brachycephalic breeds can be awkward, and again a laryngoscope makes a huge difference. Having a wide array of tube sizes available (five or more tubes of varying size) is generally the best option in these cases. An 8F U-cath with a 3.5 Portex ET tube connector can be connected in order to provide O2 and to use as a guidewire for difficult intubations.
Remember to assess your patient immediately after intubation. Check that the patient is breathing and that they have a good pulse (learn to palpate the pulse over the dorsal pedal artery as this gives a better indication of peripheral perfusion than the femoral artery) before progressing to other tasks.
Maintenance
During the maintenance period good monitoring is key. Making sure everything else for the procedure is organised prior to induction (surgical kit, suture material etc) will enable your nurse to spend more time monitoring the patient and less time fetching things during anaesthesia. In order to minimise the adverse effects of inhaled anaesthetics try to keep the vaporiser setting as low as possible this is made easier by providing appropriate analgesia. The maintenance period is made easier by planning for the likely complications. Most texts describe the administration of IV fluids during the maintenance of anaesthesia.
Reasons put forward for the administration of IV fluids are:
- The fasted patient has preoperative fluid deficits.
- Insensible fluid losses via the respiratory tract and surgical wounds are increased.
- There is a large shift of fluid into the “third space”.
- Preload needs to be maximised by fluid therapy in order to maintain cardiac output and blood pressure in the face of anaesthetic related vasodilation.
- Urine production needs to be maintained during the perioperative period.
- Fluid therapy is required in order to treat intraoperative haemorrhage.
- Fluid therapy is required to maintain tissue oxygenation in the perioperative period.
- Hypervolaemia is harmless and the kidneys regulate any fluid excess.
Whether all of the above reasons are accurate has not been fully investigated but nonetheless fluid therapy is generally recommended for most moderate to long anaesthesia. 5ml/kg/h of Hartmann’s solution for minor surgical procedures and 10ml/kg/h of Hartmann’s for major surgery is likely to be a safe and adequate dose in most animals but in animals with heart disease (especially cats) even this may be too much.
Recovery and the post operative period
Don’t forget to monitor your patients in the post operative period. 50% of perioperative mortalities in dogs and 60% in cats occur post-operatively, a majority of which occur within 3h of extubation (CEPSAF 2008). This is probably due to a tendency for animals to receive reduced monitoring during this period.
In the immediate post op period animals...
- go from breathing 100% O2 to room air very rapidly
- are often still under the influence of anaesthetic agents and still have marked cardiovascular and respiratory depression
- are cold and often shivering which increases O2 demand potentially leading to hypoxaemia
- often go from having constant monitoring to having intermittent monitoring/observation
A few examples of things can be done to counter these effects...
- Continue to warm patients on recovery until they are fully awake
- Try to instigate a more formal post operative monitoring system - i.e. encourage the continuation of the anaesthetic record into the recovery period
- Pulse oximeters can be left on patients at least until extubation. I would argue that in a healthy animal they are more useful post anaesthesia than they are intra-operatively if the animal is breathing >50% O2!
- Provide supplemental O2 to sick or shivering animals until they are fully awake animalsor if an animal has a SpO2 <93%.
Post operative analgesia
One of the biggest problems facing the clinician trying to administer appropriate analgesia is identification and quantification of pain in different individuals and moreover different species. This becomes more difficult when different people are assessing the animals. One option is to use pain scoring techniques. The short form of the Glasgow composite pain score for dogs has been shown to be relatively robust and repeatable, with minimal inter-observer variability. Reassessing patients at regular intervals is the best way to keep an animal’s analgesia at an appropriate level so getting the nursing staff involved in the pain assessment is useful as they generally spend more time with the patients and are very vigilant when it comes to changes in a patient’s behaviour.
Conclusion
There are no short cuts in anaesthesia and there is no single tip that will make anaesthesia safer and less stressful apart from being prepared and being vigilant. There are however a number of minor improvements that can be made which all add together to improve the situation for your patients and for you!
This article was kindly sponsored by Midmark, manufacturers of veterinary anaesthesia equipment:
Further reading:
Brodbelt DC, Blissett KJ, Hammond RA, Neath PJ, Young LE, Pfeiffer DU, Wood JLN. The risk of death: the Confidential Enquiry into Perioperative Small Animal Fatalities (CEPSAF). Veterinary Anaesthesia and Analgesia 2008; 35:365–373- the biggest and most recent study into peri-anaesthetic risk, contains details all small animal vets should be aware of
Seymour C, Duke T (editors). BSAVA Manual of canine and feline anaesthesia and analgesia 2nd edition. 2007, BSAVA Publishing - a good affordable basic text for small animal anaesthesia
Dugdale A. Veterinary Anaesthesia; Principles to Practice. 2010, Wiley-Blackwell- an alternative to the BSAVA manual which also covers large animals
* This article was previously published in September 2012, before veterinary licenced methadone products were available although the content is still relevant it is worth noting that licenced methadone products are now available.

.jpg)

